Welcome to my “Entertaining Chemistry” blog. My name
is Aiym Bigazy. I am a chemistry major student at Nazarbayev University. I am
interested in chemistry, because chemistry is a central science; hence other sciences, particularly physics and
biology, are tightly connected with it. Moreover, chemistry is indefeasible
part of our lives, as every object surrounding us has a chemical nature. In
this blog I would like to introduce chemical nomenclature, which is used for
naming compounds.
First of all, chemical nomenclature is a set of rules
to name the compounds established by IUPAC.
IUPAC abbreviates as International Union of Pure and Applied Chemistry. These
rules were designed because of difficulties in
naming of infinite number of compounds. These include, organic and inorganic
substances, which can be composed of various elements with different
properties, for example, metals and nonmetals, different oxidation states, etc.
 |
| Figure 1. Periodic table of chemical compounds. Source |
Let’s begin with the brief explanation of
elements in Periodic Table. Vertical columns marked as IA-VIIIA are called
groups. The elements of first group are called alkaline metals and they consist of lithium, sodium, potassium, etc. The second group elements
are called alkaline-earth metals, these are beryllium, magnesium and others.
Groups IIIB- VIIIB (red colored) are transition metals, which have several
oxidation states (charges). Beginning from IVA to VIA are nonmetals; and
Fluoride, Chloride, Bromide, Iodide, Astatine are called halogens. The last
group elements are the least reactive and called Nobel gases. Names and symbols
of all elements you can see here.
According to Zumdahl and DeCoste, there are following rules in naming compounds:
1) In
binary ionic compounds (consist of positive and negative ions), firstly, cation
(positive) is named, secondly, anion (negative) is named. Cations are usually
metals, anions are nonmentals.
Metal + Nonmetal + ide suffix
If metal forms
more than one cation (with different oxidation states), then Roman numeral
showing the oxidation state of cation is added. For example, NaCl is called sodium
chloride, because sodium has charge +1 only, whereas CuCl2 is called
copper (II) chloride, because copper can have charge +1 and +2, in this case it
is +2.
Metal + Roman numeral + Nonmetal + ide suffix
2) If
a compound contains more than two elements it is called polyatomic, they are
named in the same manner, but names of polyatomic ions are also established by
IUPAC and should be memorized. For example, Na2SO4 is
sodium sulfate, Mg(OH)2 is magnesium hydroxide; here is the list of polyatomic ions.
3) Binary
covalent compounds consist of two nonmetals, but their names are similar with
ionic compounds. However, there are some rules that should be applied to naming
covalent compounds:
- Firstly, the element at the beginning of the formula is named (cation)
- Secondly, negative ion is named, which is the second element
- When
a compound contains two nonmetals or more, to determine the number of atoms of
each element prefixes are used:
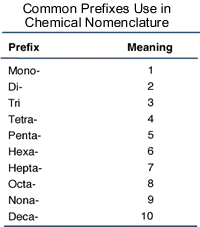
Figure 2. Prefixes determining the numbers.
Source
For example, P2O5 is called diphosphorus
pentoxide, and N2O4 is dinitrogen tetroxide.
Moreover, there is more complicated system of naming
organic compounds and due to the word limit it will not be covered in this
blog.
Summary:
 |
| Figure 3. Naming chemical compounds. Source |
References
"Formula, Name." http://www.ars- chemia.net/Classes/68/Notes/List_of_polyatomic_ions.pdf.
http://www.ars-chemia.net/Classes/68/Notes/List_of_polyatomic_ions.pdf
(accessed September 30, 2014).
"Welcome to the International Union of Pure and Applied Chemistry."
IUPAC. http://www.iupac.org/ (accessed
September 30, 2014).
Zumdahl, Steven S., and Donald J. DeCoste. Chemical principles. Seventh
ed. Brooks: Cengage Learning, 2013.
Комментариев нет:
Отправить комментарий