Dear
readers!
Here is my
new blog post, which is related to the recent discovery in the field of
chemistry. This blog is devoted to the discovery of new super-heavy element
Ununseptium.
By the end
of this blog you will know:
1) When and by whom the element was
discovered.
2) The process of synthesis of new
element.
3) What is the worldwide significance and
the limitations of the element.
History of 117 element
 |
| Source |
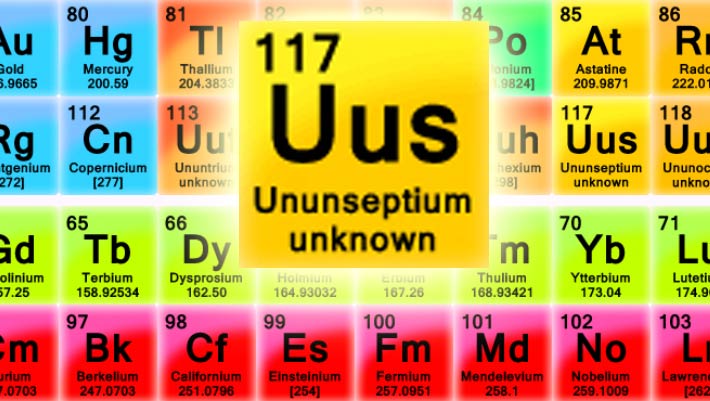
Element 117
of periodic table was firstly discovered in 2009 by researchers from Flerov
Laboratory of Nuclear Reactions in Russia, but synthesis of new atom was
declared invalid. The element obtained
recognition only in 2010, with efforts of international team hosted by
Professor Christoph Dullmann and
collaboration of 72 scientists from different countries in the GSI Helmholtz
Center for Heavy Ion Research, Germany.
The process of discovery
First of
all, Ununseptium belongs to the super-heavy elements, because it has atomic
number 117. All elements that have atomic number greater than 103 are called super-heavy.
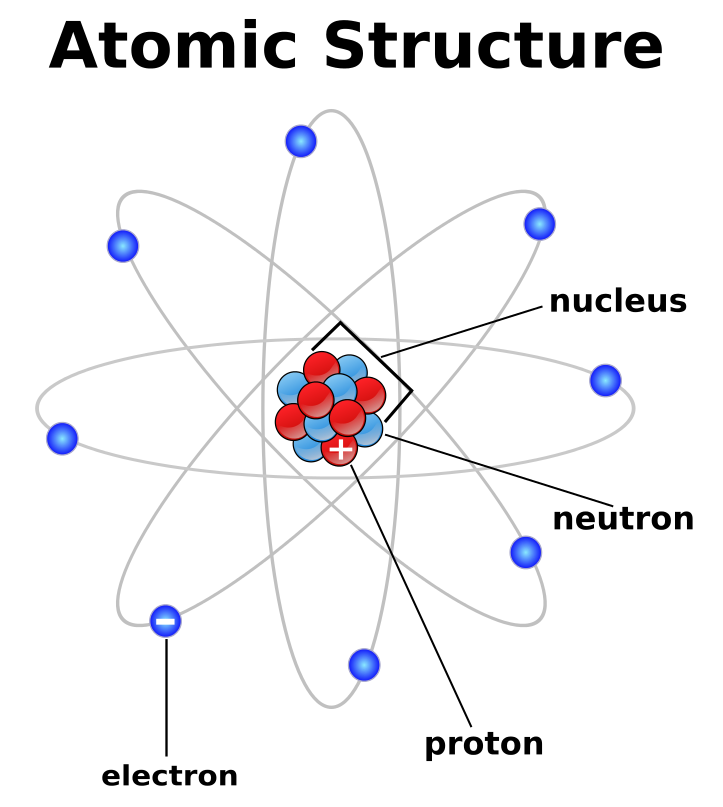
Atomic number indicates the number of protons (positively charged particles),
along with protons atom contains electrons (negatively charged particles) and
neutrons (neutral). Structure of the atom is represented by nucleus made of protons
and neutrons, and electrons moving around the nucleus.
 |
| Source |
Ununseptium
element does not exist naturally; consequently,
it was synthesized by fusion of nuclei of two different elements. In other
words, by adding protons from nucleus of one element to the nucleus of another
element. In that experiment, calcium-48 and
berkelium-249 isotopes were used.
Berkelium-249 was bombarded with calcium-48. Particularly, calcium ions
were accelerated and these high speed ions crashed into berkelium-249 resulting
in ununseptium-294 isotope (some protons were lost during bombarding). In order
to understand this concept better, imagine two snowballs. One snowball, which
has high speed bumps another one, which causes merging of these two snowballs. This fusion
can be accompanied by splitting off small particle of the snowball.
However,
actual experiment is more complicated and was performed in the special GSI
accelerator, more information about it you can find here.
 |
| Source |
Significance and limitations
The most
important achievement of that discovery is that it demonstrated that the
science does not depend only on naturally occurring elements. Which means, that
people can make new elements from already existing and create other super-heavy
elements. Nevertheless, the main
disadvantage of super-heavy elements is that they have very short half-life.
Half-life is the time, which is required to atom to decay, or when only the
half of the atom remains. This means, that such super-heavy elements do not
exist for a long time and cannot be used in industry currently. Moreover, synthesizing
elements by using this method requires large energy expenditures.
To sum up, in
this blog I have explained the discovery of 117 element of Periodic table,
Ununseptium, and what is the importance of that discovery. Furthermore, the
main limitation of super-heavy elements was given.
References
Chemical
half-life. (n.d.). The Free Dictionary. Retrieved October 30, 2014,
from http://www.thefreedictionary.com/Chemical+half-life
Element 117, discovered by
Laboratory, one step closer to being named. (2014, May 2). Lawrence Livermore
National Laboratory. Retrieved October 30, 2014, from https://www.llnl.gov/news/element-117-discovered-laboratory-one-step-closer-being-named
Isotope. (n.d.). Dictionary.com. Retrieved October 30,
2014, from http://dictionary.reference.com/browse/isotope
Newly discovered element 117 is a
step toward useful super-heavy elements | ExtremeTech. (n.d.). ExtremeTech. Retrieved October 29,
2014, from
http://www.extremetech.com/extreme/181807-new-element-117-is-a-step-toward-useful-super-heavy-elements
PRESS AND PUBLIC RELATIONS. (n.d.).International
team of researchers observes new superheavy element 117. Retrieved October
30, 2014, from http://www.uni-mainz.de/presse/17241_ENG_HTML.php
Scientists Discover New Element:
Ununseptium, The Heaviest Element Ever Made - From Quarks to Quasars. (n.d.).From
Quarks to Quasars. Retrieved October 30, 2014, from
http://www.fromquarkstoquasars.com/scientists-discover-new-element-meet123/
Superheavy. (n.d.). Dictionary.com. Retrieved October 30,
2014, from http://dictionary.reference.com/browse/superheavy
Ununseptium Element Facts. (n.d.).Chemicool.
Retrieved October 30, 2014, from
http://www.chemicool.com/elements/ununseptium.html